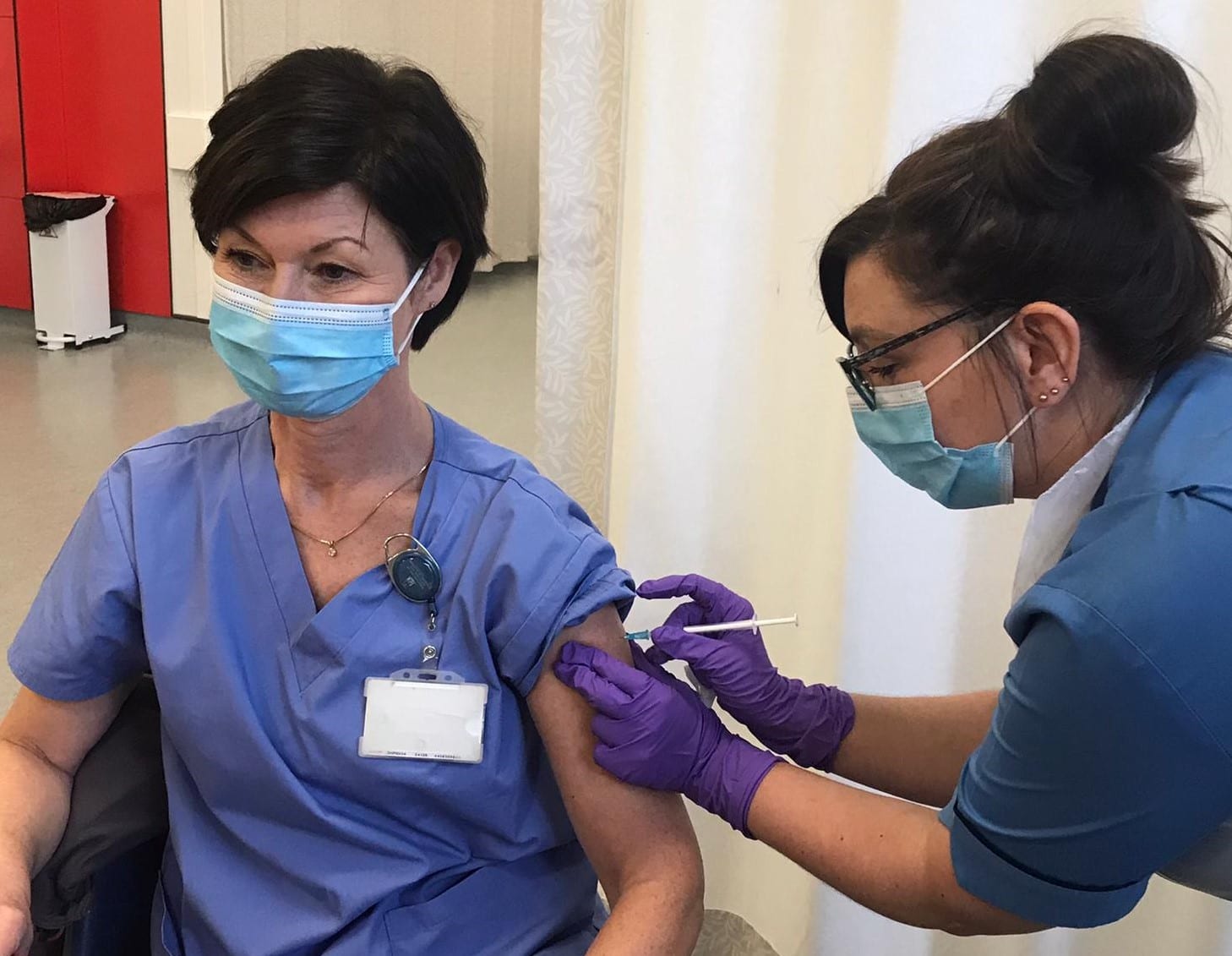
In November 2020, the Center for Genetic Engineering and Biotechnology of Cuba launched a trial on a coronavirus vaccine called Abdala. The Abdala vaccine consists of a piece of the coronavirus spike protein called the receptor binding domain, and is delivered in three doses. On Feb. 1, the center held a press conference to announce the start of a Phase 2 trial. A Phase 3 trial involving up to 48,000 participants was launched on March 18. On May 12, while the Phase 3 trial was still underway, the Cuban government began rolling out Abdala in a mass vaccination campaign, in the hopes of reining in a surge of cases.
On June 21, Cuban officials reported that Abdala had an efficacy of 92.28 percent. Cuban officials approved a trial for Abdala in young people on July 1. The Cuban government granted emergency use authorization for the vaccine on July 9.
Abdala is one of two Cuban vaccines tested in a Phase 1/2 clinical trial to assess their ability to increase immunity in those who have already had Covid-19. Sinovac conducted phase 3 trials involving volunteers in Brazil, Indonesia and Turkey. Although it is not yet approved by regulators, shipments have already arrived in Indonesia, ready for rollout. A report in July said that the Chinese government has given the Sinovac vaccine emergency approval for limited use. The city of Jiaxing has reportedly offered the vaccine to health workers and other high-risk groups for US$ 60.
A phase 2 study was carried out on 12 July 2021 to determine the safety and immunogenicity of booster doses. A phase 3 study is due to be conducted in late August 2021 to evaluate the efficacy of the vaccine in participants aged 6 months to 17 years. A study report on a phase 4 clinical trial was published on 23 August 2021 which found that simultaneous administration of the vaccine and seasonal influenza vaccine would be feasible. In December 2020, the UK became the first country in the world to approve this vaccine and began rolling out an initial 800,000 doses at the start of the month, and is now in use worldwide.
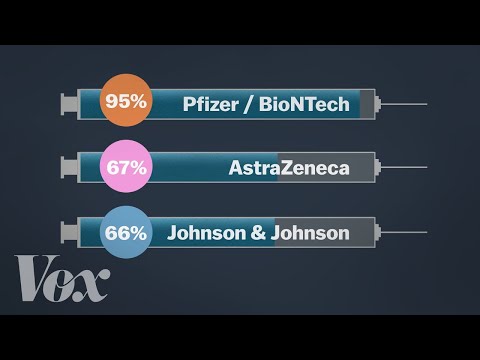
BioNTech, working together with Pfizer, started testing its BNT162 vaccine in humans in global trials, initially in Germany, and then started trials in the USA. BioNTech has also entered into a € 100 million debt financing agreement with the European Investment Bank in order to scale-up the production of the vaccine in Europe. On 27 July 2020, it announced the launch of a phase 2/3 trial with 30,000 volunteers in the USA and other countries including Argentina, Brazil and Germany.
In September, it said it would expand its phase 3 US trial to 44,000 participants. At the start of October 2020, BioNTech and Pfizer started recruiting for a phase 3 trial in South Africa and by early November had reported promising interim results. In February, a phase 4 trial was launched as part of a national cohort study in collaboration with the Danish Ministry of the Interior and Health. The United States Food and Drug Administration has authorised its use for 12 to 15 years olds in the United States. On 23 August 2021, the FDA granted full approval to the vaccine for people 16 and older, moving it beyond the emergency-use status in the US – the first vaccine to do so.
A phase 2 study was carried out in August 2021 to elicit an antibody response in kidney transplant recipients who have failed to respond to two doses of vaccine. On 12 August 2021, the United States FDA authorised the use of booster shot in transplant recipients. On 27 September 2021, a phase 4 trial was registered to study a third dose of the vaccine to boost COVID-19 immunity. The company started the third phase of its clinical trials in July 2020. Preliminary findings from its phase one trials showed that healthy subjects—including elderly patients—produced coronavirus antibodies and a reaction from T cells, another part of the human immune response. The company also announced plans to test the safety and efficacy of a booster shot that would be delivered a year after the first pair of vaccine doses, according to CNBC.
The COVID-19 vaccine was jointly developed with the Institute of Biotechnology of the Academy of Military Medical Sciences. On 25 June 2020, the Chinese military approved the vaccine for a year as a "specially needed drug", which is unusual given that at that point phase 2 results had not been collated. On 9 August 2020, the Saudi health ministry announced that CanSino would run a phase 3 trial in Saudi Arabia; later in the month the company also started a trial in Pakistan and Russia.
In May 2021, the company registered a phase 4 trial with 300 adult participants who have been primed with either one or two doses of inactive SARS-CoV-2 vaccine. On 5 August 2021, Reuters reported that antibody levels in people inoculated with this vaccine could drop to around 30 percent after six months. A phase 2 trial report published on 22 September 2021 confirmed a single dose of the vaccine was safe and induced robust immune responses in individuals aged 6-17 years. When candidate vaccines make it to human clinical trials, they first go through phase 1 trials primarily to test the vaccine's safety, determine dosages and identify any potential side effects in a small number of people.
Phase 2 trials further explore safety and start to investigate efficacy on larger groups. The final stage, phase 4 trials, is conducted after national regulatory approval and involves further monitoring in a wide population over a longer timeframe as a form of post-marketing surveillance . However, not all vaccines that have been approved for domestic are in phase 4 trials.
Regulators in many countries have their own individual procedures and timelines for providing emergency use authorisations, relying on various types of evidence at different clinical trial phases. Some national regulators, including those in Russia and China, began approving vaccines for public use even before phase 3 trials were completed. The World Health Organization lists candidates at various stages of clinical trials.
This vaccine candidate which uses self-amplifying RNA technology has been developed by researchers at Imperial College London. The research has received £ 41 million in funding from the UK government. The clinical trial in the current phase will involve administration of the two-dose vaccine to 300 healthy individuals. Volunteers have already started receiving the first dose of the vaccine. Imperial College has also created VacEquity Global Health, a social enterprise, to develop and distribute low-cost vaccines around the world, including to low- and middle-income countries.
However, in January Imperial College announced that it will not be proceeding with further efficacy trials in the UK, beyond those currently underway, especially given the number of vaccines already approved and in use in the country. Rather, it will now focus its efforts on making its existing candidates work as powerful booster vaccines against emerging and longer-term coronavirus threats, including mutations and variants. On March 23, CanSino announced that it had won approval for a clinical trial of an inhaled version of the vaccine. Chief Executive Yu Xuefeng said on June 2 that this version is now in Phase 2 trials. Preliminary findings from the Phase 1 trial suggest that the inhaled vaccine was effective at stimulating an immune response, and that an injected dose followed by an inhaled dose may yield the best results. Researchers have also begun to test whether giving alternating doses of vaccines from CanSino and Anhui Zhifei Longcom can boost their effectiveness.
On June 9, Reuters reported that the CanSino vaccine is being tested as a booster shot. Results from a Chinese study, released on Sept. 7, suggested that getting the CanSino booster shot after the Sinovac vaccine produced a stronger antibody response compared to a third shot of the Sinovac vaccine. CanSino announced on Sept. 26 that a trial in children age 6 to 17 yielded positive results. Starting in August 2020, CanSino began running Phase 3 trials in a number of countries, including Pakistan, Russia, Mexico and Chile.
On Feb. 25, China announced the approval of the CanSino vaccine for general use. The company announced that its one-shot vaccine had an efficacy rate of 65.28 percent at preventing all symptomatic Covid-19 cases. But on April 1, CanSino's chief scientific officer said that the efficacy of its vaccine could drop over time.
He also floated the idea of using a booster shot six months after the first dose, though more clinical trial data is needed. Reuters reported on Aug. 5 that this drop could be as much as around 30 percent after six months. We announced on September 28 that our COVID-19 recombinant vaccine candidate is progressing in a phase 3 efficacy and safety trial. In parallel, we have expanded our research program to include a study of the vaccine as a booster dose.
Recently published preclinical data indicated that the vaccine candidate has the potential to strongly boost immune response following vaccination from many of the currently approved vaccines and against a broad range of variants. On June 1, Moderna announced that it has applied for full FDA approval of its COVID-19 vaccine for use in people age 18 and older. The company also plans to file for emergency use authorization for teens ages 12 to 17. A study of clinical trials among adolescents in this age group shows that its vaccine is safe and 100 percent effective. The study also showed the vaccine is 93 percent effective among participants in this age group two weeks after the first dose.
It was developed by the University of Oxford, and has been granted emergency use authorisation by the European Medicines Agency, the WHO as well as national regulators worldwide. This vaccine is fridge-stable meaning that it can be easily transported anywhere in the world. At about US$ 4 per dose for lower-income nations, it is also being made available for a fraction of the cost of others . It was tested in phase 3 clinical trials with more than 10,000 people from across the UK, including children and older people. The vaccine was also tested in Brazil, the United States and India and South Africa started the first COVID-19 vaccine trial in Africa. In February 2021, a phase 4 trial was launched as part of a national cohort study in collaboration with the Danish Ministry of the Interior and Health.
A phase 2/3 trial was started on 1 June 2021 to study the effectiveness, safety and immunogenicity of a half dose of the vaccine. On 27 September 2021, the company registered a phase 4 trial to assess the immunogenicity and safety of the vaccine for prevention of COVID-19 in immunocompromised adults. On Sept. 11, 2021, COVAXX registered a Phase 1 trial in Taiwan which led to 100 percent of volunteers producing antibodies without any serious side effects.
A Phase 2/3 trial is planned to launch in Brazil, India and other countries. On Nov. 25, Covaxx announced agreements with countries including Brazil, Ecuador, and Peru to deliver more than 140 million doses for $2.8 billion. In January, the company announced they were also starting preclinical research on a vaccine tailored specifically to newly emerged coronavirus variants that could potentially evade conventional vaccines. In a June 21 press release, Vaxxinity said it expected to deliver the vaccine by the end of the summer, which did not come to pass. The researchers registered a Phase 1 trial on July 20 to assess the effectiveness of a third dose of its vaccine in those who have already received two.
But in August, Taiwan regulators rejected an application for emergency approval of the vaccine after it failed to meet their standards on neutralizing antibodies. Last spring, researchers at the University of Washington developed a nanoparticle studded with pieces of the coronavirus spike protein. The South Korean vaccine company SK Bioscience licensed the vaccine, called GBP510. After partnering with GSK, they launched a Phase 1/2 trial of the vaccine in February 2021. It is the second vaccine SK Bioscience has put into trials, after launching a study on another protein-based vaccine called NBP2001.
SK Bioscience has received $210.1 million from the Coalition for Epidemic Preparedness Innovations for the development of GBP510. In August, the company launched a Phase 3 trial, comparing GBP510 to AstraZeneca's Vaxzevria vaccine. Before the pandemic, the Pennsylvania-based company Inovio developed DNA-based vaccines that are delivered into the skin with electric pulses from a hand-held device. They are running clinical trials for vaccines against a number of diseases, including HIV, Zika, and several forms of cancer. At the start of the pandemic, Inovio developed a DNA vaccine against the spike protein on the coronavirus.
A Phase 1 trial, published in December, did not uncover any serious adverse effects, and measured an immune response in all 38 volunteers. In collaboration with the US BARDA, this vaccine candidate uses our recombinant protein manufacturing platform together with GSK's pandemic adjuvant platform. In parallel, we have expanded our research program to include a study of the vaccine as a potentially broadly protective booster dose. Recently published preclinical data showed that it has the potential to strongly boost immune responses following vaccination with many of the currently approved vaccines and against a broad range of variants.
On 4 August 2021, to reduce unequal distribution between rich and poor countries, the WHO called for a moratorium on a booster dose at least until the end of September. However, on 18 August, the United States government announced plans to offer booster doses 8 months after the initial course to the general population, starting with priority groups. Before the announcement, the WHO harshly criticized this type of decision, citing the lack of evidence for the need for boosters, except for patients with specific conditions. At this time, vaccine coverage of at least one dose was 58% in high-income countries and only 1.3% in low-income countries, and 1.14 million Americans already received an unauthorized booster dose. US officials argued that waning efficacy against mild and moderate disease might indicate reduced protection against severe disease in the coming months. Israel, France, Germany, and the United Kingdom have also started planning boosters for specific groups.
It is also 85 percent effective in people 65 and older and 100 percent effective at preventing severe cases of the disease. The Finlay Vaccine Institute in Havana have developed a single-dose vaccine called Soberana Plus, which is its third candidate currently in clinical trials. Like its other candidates, Soberana 1 and Soberana 2, Soberana Plus contains a part of the spike protein, called RBD, along with adjuvants to boost an immune response to COVID-19. Unlike the other two, Soberana Plus is specifically targeted at people with a history of COVID-19 infection. The Institute's Soberana 1 vaccine is currently registered for a phase 2 clinical trials with 450 participants.
Gamaleya has started a phase 1 clinical trial on this non-replicating viral vector vaccine candidate with two sets of volunteers receiving vaccination since mid-June. Before the vaccine went on to later trials Russiaannounced that it would be approved for use. A phase 3 trial of the vaccine began with more than 2,000 people in Russia, Latin America and the Middle East, and then expanded to 40,000. On 2 February, the company published the results of their phase 3 trial. A study published in July found that Sputnik antibodies can neutralise the Delta variant, although not as effectively as with the original virus.
Researchers at City of Hope, a California biomedical research institute, created a vaccine based on a weakened form of a virus called Modified Vaccinia Ankara, or MVA for short. They added two coronavirus genes to the MVA virus — one for the spike protein, and one for another protein called nucleocapsid. They are testing the vaccine specifically for people with immune systems impaired by cancer and other disorders.
Many of them do not produce a strong immune response to authorized vaccines based on mRNA. On Nov. 24, 2020, City of Hope announced the start of a Phase 1 trial. In September 2021, researchers launched a Phase 2 trial, giving the vaccine to volunteers with blood cancer who have received a bone marrow transplant or a form of immunotherapy called CAR-T.
The company also said that real-world evidence and data from its clinical trials show that its single-dose vaccine remains effective against COVID-19. The press release cited a study published to a preprint server, which shows that the vaccine remains 79 percent effective in preventing COVID-19 and 81 percent effective in preventing hospitalizations. Johnson & Johnson said these data are consistent with findings from its clinical trials, which showed 75 percent efficacy against severe disease and 89 percent efficacy against hospitalization.
The Beijing Institute is part of China's state-run Sinopharm Group, and developed its vaccine called BBIBP-CorV, in collaboration with the Chinese Center for Disease Control and Prevention. A phase 3 trial is due to be conducted in October 2021 to assess the safety, immunogenicity and efficacy of two doses of the vaccine, followed by a booster dose in adults 18 years of age and older. Researchers at the Institute of Medical Biology at the Chinese Academy of Medical Sciences, which has invented vaccines for polio and hepatitis A, created an inactivated coronavirus vaccine. In May 2020, they launched a Phase 1 trial on 192 volunteers which indicated the vaccine was safe and produced an immune response.